

The enthalpy of a reaction, AH sys (also called AHræn), also has an influence on the spontaneity of that reaction. If either the ASsys or AS surr is a positive number, its contribution is considered a driving force for spontaneity. The entropy change of the universe is the total entropy change of the system and the surroundings: AS universe = AS system + AS surroundings Driving Forces for Spontaneity Although the conditions below are driving forces for spontaneity, it is ONLY the combination of the two conditions what determine whether a process will be spontaneous.
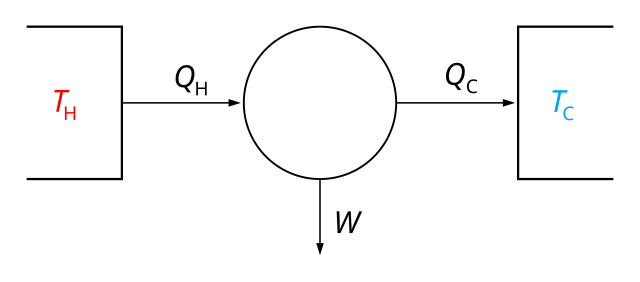
Transcribed image text: The Second Law of Thermodynamics states that the entropy of the universe, Suniv, is always increasing because the AS univ is positive for all spontaneous processes.


 0 kommentar(er)
0 kommentar(er)
